
AI Act – what impact will it have on devices incorporating artificial intelligence systems?
Do you manufacture devices incorporating artificial intelligence systems and wonder how the newly published AI Act will impact you? Read the rest of this article 😉
The key message to remember: The MD and IVD Regulations continue to drive DM/IVDs incorporating AI!
-
-
-
- The AI Act is based on a ‘horizontal’ approach, not specific to a particular sector of activity.
- Unlike MDR/IVDR, which are based on a ‘vertical’ sectoral approach to regulation, specific to a type of product.
-
-
This means that, for a device incorporating an AI system :
-
-
-
- AI-specific aspects and requirements are defined by the AI Act
- The overall safety of the final product is covered by the MDR/IVDR
- The compliance of AI systems with the requirements of the AI Act will be assessed as part of the conformity assessment already provided for by the MDR/IVDR
-
-
As with the MDR/IVDR, the AI Act classification is based on the product’s level of risk:
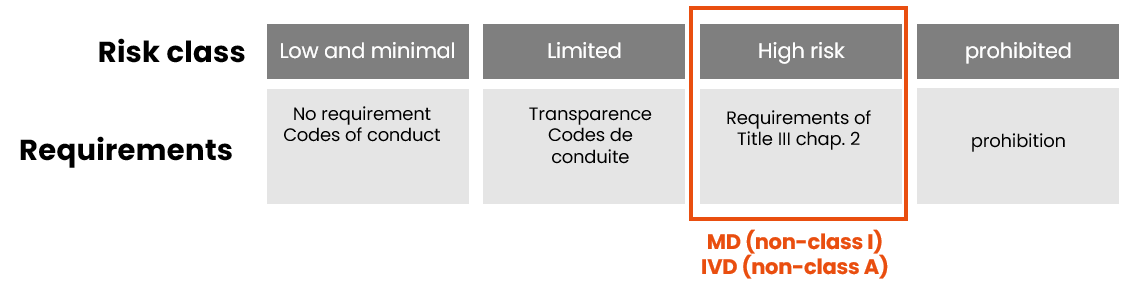
Remember that devices incorporating an AI system (except Class I MD and Class A IVD) are automatically classified as high-risk AI systems under the AI Act, regardless of their risk class under the MDR/IVDR. This high-risk classification leads to a systematic review of the technical documentation during the conformity assessment.
However, there are 2 exceptions:
-
-
-
- class I medical devices (which are not sterile, have no measuring function and are not reusable surgical instruments) and non-sterile class A IVD
- legacy devices
-
- which are not high-risk AI systems and are not subject to a conformity assessment procedure under the AI Act.
- But which must still apply the requirements of Title IV of the AI Act: the obligation to be transparent with data subjects about the fact that they are interacting with an AI system, unless it is obvious.
-
-
-
Many requirements are common to both texts (MDR/IVDR and AI Act).
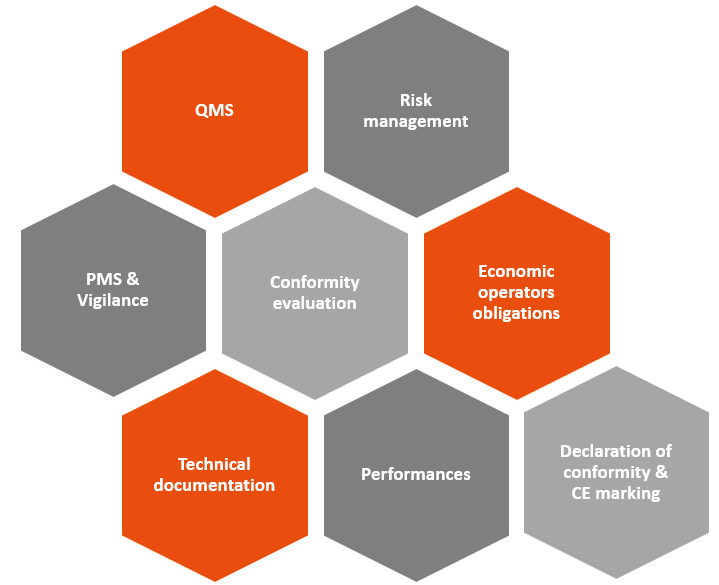
But… there are also a few new features that will impact manufacturers of devices incorporating AIS, including:
-
-
-
- Data governance: High-risk AIS must be developed using high-quality datasets for learning, validation and testing.
- Human control: High-risk AIS must be designed to allow humans to supervise them effectively using appropriate human-machine interface tools.
- Accuracy, robustness and cybersecurity: which must be maintained throughout the lifecycle of high-risk AIS.
-
-
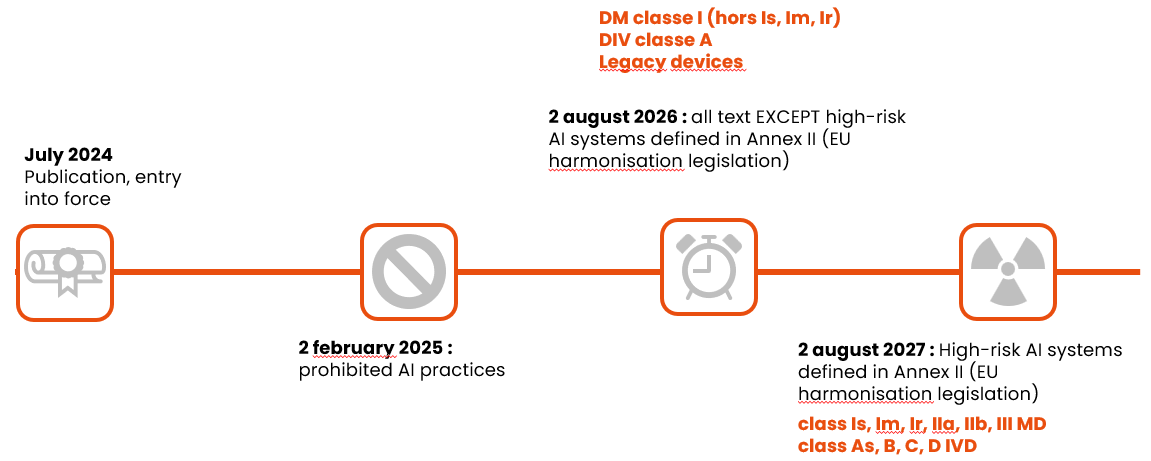
And finally, here are the application dates to remember, depending on the class of device:
If you would like to find out more:
-
-
-
- A webinar on the relationship between the AI Act and the MDR/IVDR was recently hosted by nexialist.
- Check out our podcasts.
- Our section about AI with all regulatory texts, standards, or guides on an international scale.
-
-
En savoir plus sur le monde du DM & DMDIV
Nos articles récents
Liability for defective product Directive (EU) 2024/2853: what’s new for MD and IVD manufacturers?
On the eve of its 40th anniversary, the Liability for defective product Directive is being renewed to adapt to technological innovations, combat legal inconsistencies and ensure legislative consistency within the EU. Published in the Official Journal of the European...
Switzerland: the ivDO amended to adapt to the extension of European transitional provisions & future developments!
The Swiss Ordinance on in vitro diagnostic medical devices (IvDO) was amended by Federal Council Decision RO 2024 741 of 20 November 2024 (link in French), which came into force on 1 January 2025. So what are the changes brought about by this revision at the start of...
UE – MDCG 2024-15 – Guidance on the publication of the clinical investigation reports and their summaries in the absence of EUDAMED – November 2024
Theme Clinical investigation report Target Audience Sponsor, competent authorities, Commission Products concerned MD Documents mentioned MDR (EU) 2017/745 – Art 77 MDCG 2021-1 Rev1 This guide explains the procedure for publishing clinical investigation reports...



0 Comments