
6 Dec 2019 | All of our solutions
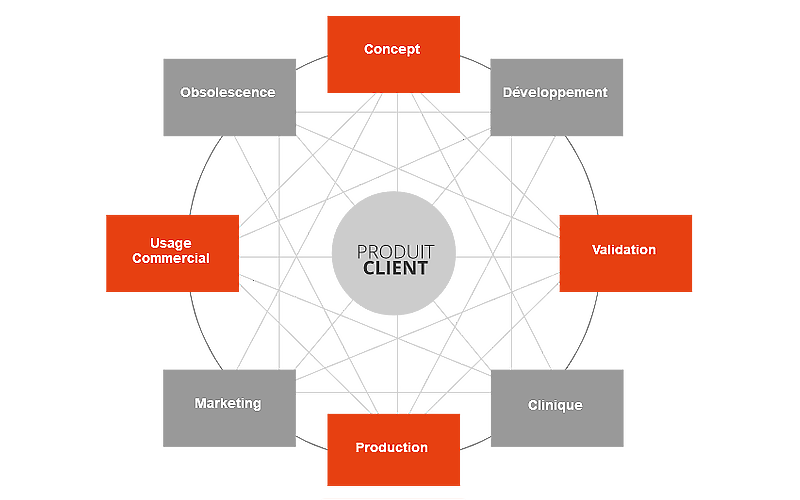
Clinical assessment and investigation During your product development, you reach the clinical assessment stage, but how do you address this complex problem? Nex’Med has the solution. A simple and accurate service takes the form of a comprehensive clinical...
6 Dec 2019 | All of our solutions
Post-market surveillance PMS/ PMCF: You recognise these words, and they may be sometimes familiar, but you do not really know what they mean. You are not sure what information to supply. What level of detail and analysis is expected? Are you looking for a...






